Waking up the Sentinel and Enhancing Defendence against Invaders
—CAP, Preventing Shrimps from the Infection of WSSV
The mechanism by which CAP combats White Spot Syndrome Virus (WSSV) in shrimp primarily involves activating the Toll signaling pathway and enhancing immune responses, with the specific process as follows:
PART I, Toll Signaling Pathway (TSP)
As an evolutionarily conserved signaling pathway located in cell membrane and endosomal membrane, TSP is the core mechanism by which organisms recognize pathogens and initiate immune responses, essentially the body’s “alarm system”.
Basically, a TSP usually includes the following important proteins:
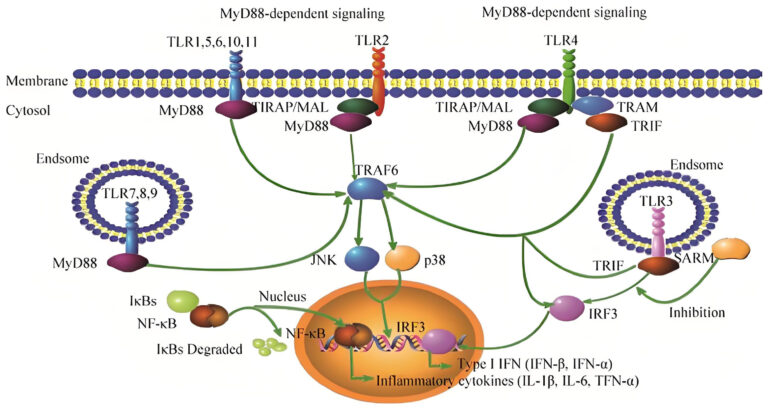
Core proteins :Toll-like receptor (TLR), MyD88, TRIF
Auxiliary proteins: IRAK4/IRAK1, TRAF6, TBK1/IKKi
Regulating proteins:TRAF3, RIP1
Other related proteins: MD-2, CD14
To summary, these proteins together form the core framework of the Toll signaling pathway, regulating the initiation and balance of immune responses through complex interactions.
signaling pathway, regulating the initiation and balance of immune responses through complex interactions.

1.1 TSP of Shrimps
Shrimp blood cells and plasma can secrete antimicrobial peptides as one of its natural immune weapons. The TSP in shrimp is the core of its immune defense, which protects shrimp health by recognizing pathogens to activate its own antimicrobial peptides and promote the production of interferon. The specific mechanism is as follows:
1.2.1 Key component proteins and functions/signal transmissions.
1.2.1.1Toll receptor
Structure: Containing LRR (leucine enriched repeat) and TIR (Toll/IL-1 receptor) domains.
Function: Toll receptors act like “sentinels” and can directly or indirectly identify pathogen associated molecular patterns (PAMPs), such as bacterial lipopolysaccharides (LPS) or viral double stranded RNA (dsRNA), and initiate immune responses. Once a threat is detected, it immediately triggers an inflammatory response and the production of antimicrobial peptides.
1.2.1.2 MyD88 dependent pathway
Most Toll receptors transmit signals through the MyD88 protein, triggering inflammatory responses.
Core proteins: MyD88, IRAK4, TRAF6, NF – κ B.
Function: TLR4/5 activates AP-1 and NF – κ B through MyD88, inducing the expression of inflammatory factors such as IL-1 β and TNF – α, and enhancing the production of own antimicrobial peptides.
1.2.1.3 TRIF dependent pathway
Some receptors promote the production of interferon through TRIF protein, enhancing antiviral ability.
Core proteins: TRIF, TBK1, IRF3.
Function: TLR3/4 activates interferon regulatory factor 3 (IRF3) through TRIF, promoting the production of type I interferon (such as IFN – β) and inhibiting viral replication.
1.2.2 Signal transduction mechanism
After Toll receptors recognize pathogens, signals are transmitted through MyD88 or TRIF adaptor proteins.
MyD88 dependent: MyD88 recruits IRAK4, activates TRAF6, and ultimately activates NF – κ B, inducing the expression of inflammatory factors.
TRIF dependent: TRIF activates IRF3 through TBK1, promoting interferon production.
Part II. Mechanism of Activation by CAP.
In 1980, Cecropin Antimicrobial Peptide (CAP)was first extracted from a kind of silkworm pupae by Swedish scientists Boman. As an external antibacterial additive for shrimp, CAP can be one additive helping shrimp effectively enhancing its immunity under the following mechanism:
2.1 Direct binding
CAP may trigger a signaling cascade by binding to Toll receptors or their ligand.
The amphiphilic helical structure of CAP is the key to its precise ability to attack pathogens. This structure is like a “molecular key” that can directly open the defense door of bacteria. Specifically:
2.1.1 CAP’s structural characteristics for binding onto Toll receptors.
The amphiphilic helical structure of CAP (alternating hydrophilic/hydrophobic surfaces) can accurately match the binding site of Toll receptors, forming stable complexes. The hydrophilic region with a positive charge at the strongly alkaline N-terminus is responsible for “locking” the bacterial surface, while the hydrophobic region at the amidized C-terminus, acting as a “pry bar” inserted into the cell membrane, is crucial for antibacterial activities. The glycine-proline chain in the middle allows the structure to bend flexibly and adapt to the membrane morphology of different bacteria.Therefore, this structural characteristic enables it to effectively bind and activate Toll receptors.
2.1.2 Signal activation after binding.
After binding, CAP triggers a signaling cascade reaction of Toll receptors, transmitting signals through proteins such as MyD88, ultimately activating NF – κ B transcription factors and inducing antimicrobial peptide gene expression. This process significantly enhances the resistance of shrimp to WSSV.
2.2 Indirect activation
By inhibiting the infection of pathogens (such as WSSV), CAP reduces the suppression of the Toll pathway, thereby indirectly activating the pathway.
Conclusions:Application effect
Such bindings onto bacterial cell membranes through electrostatic interactions can form ion channels, leading to the leakage of contents of bacteria and bringing selective killing effects on Gram positive bacteria, Gram negative bacteria, and some cancer cells. Usually, CAP’s antibacterial spectrum covers 12 pathogenic bacteria such as Staphylococcus aureus and Clostridium difficile.
For shrimps, after activating the Toll pathway, CAP significantly increases the number of shrimp blood cells and immune related enzyme activity, reducing the mortality rate of WSSV infection.
Dosage: The addition of CAP (10-8000 mg/kg) in the feed can significantly reduce the incidence rate of enteritis and white stool syndrome.
(end and blank below)